Comprehensive Clinical Research Solutions — Now with Expert Financial & Legal Services
Ensuring precision, compliance, and seamless project execution through integrated clinical, regulatory, financial, and legal expertise across India.
25+
Clinical trials actively supported across India
Hybrid Locations
4 Cities
Ajmer, Vadodara, Mumbai, Pune
100%
Compliance with ICH-GCP and regulatory standards
Trusted by Sponsors & CROs and medical institutions for ethical and accurate research support
- About Us
Driving Precision in Clinical Research. Endorphins Research Co. delivers compliant, ethical, and results-driven support for trials across India.
Years of Experience
10+
Led by seasoned experts in trial management, quality control, and regulatory compliance.
Client Retention Rate
95%
Long-standing partnerships with clients who trust us for our reliability and high-quality services.
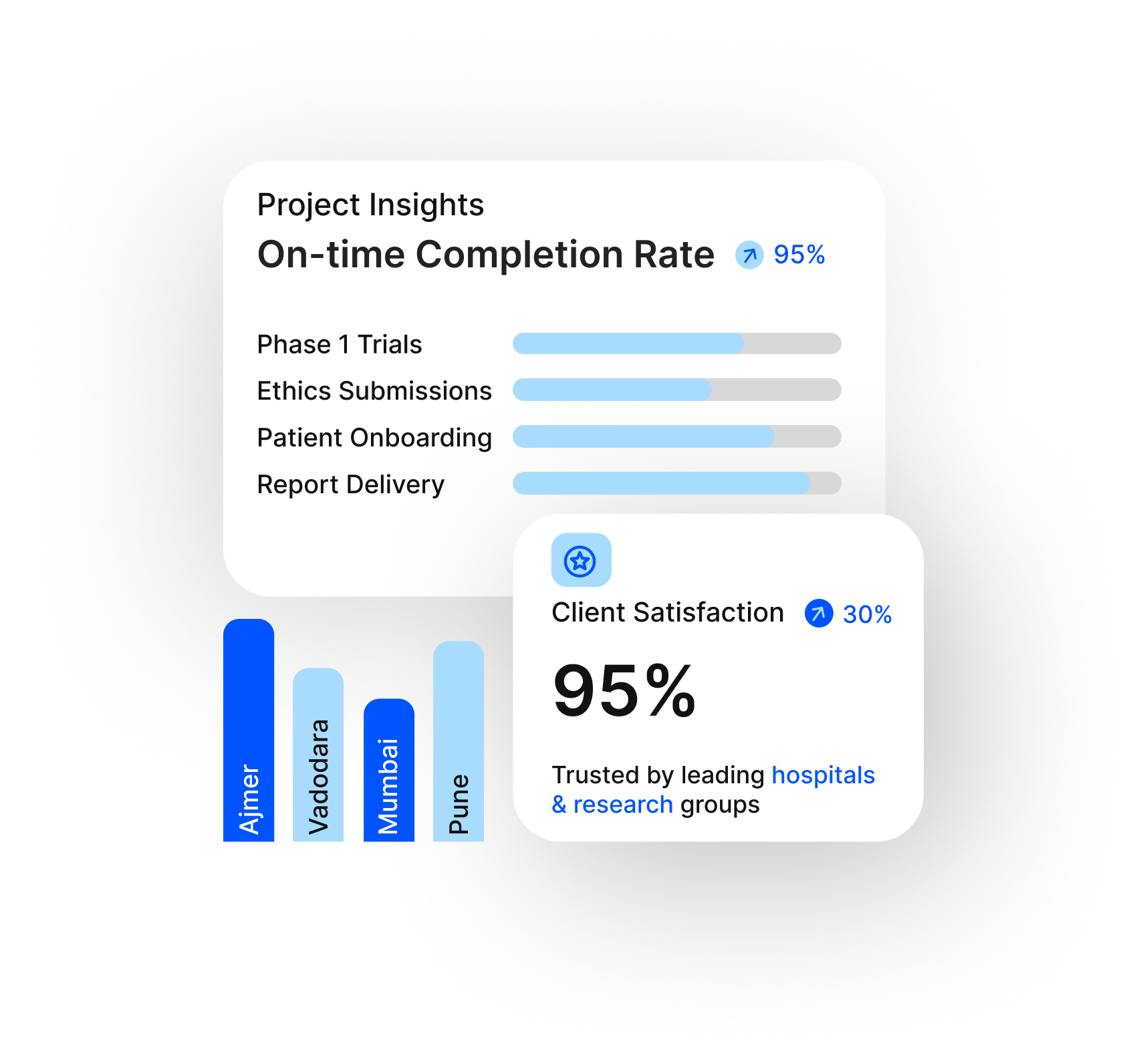
Built on Data. Powered by Trust.
We bring together clinical precision, technology, and compliance to drive successful research outcomes across India’s top metros.
Ethics-Centric Operations
Our protocols ensure ICH-GCP compliance, protecting data integrity and participant safety at every stage.
Real-Time Project Monitoring
Track milestones and performance with transparent progress dashboards and centralized reporting.
Dedicated Multicity Network
With operations in Ajmer, Vadodara, Mumbai & Pune, we ensure pan-India project delivery and patient outreach.
Expert Services, One Trusted Partner
Whether you need research support, compliance navigation, or financial guidance, our integrated service verticals ensure precision, speed, and accountability from start to finish.
Clinical Research Services
Explore our full suite of clinical trial and monitoring solutions for research excellence.
Academic & Scientific Support
From topic finalization to journal publication, we guide your academic journey.
Regulatory & Compliance Services
End-to-end compliance support for IRB, ICMR, DCGI filings and audits.
Why Choose Endorphins Research Co.?
We stand apart by combining precision, quality, and unmatched dedication in academic research and writing services.
Expert-Led Research
Every project is guided by domain experts to ensure academic integrity and precision.Trusted by 500+ Clients
Highlights
- PhD-level subject experts.
- Plagiarism-free guaranteed work.
- 100% confidentiality assured.
- Adheres to international standards.
End-to-End Support
From topic selection to final formatting — we handle it all, so you don’t have to.24x7 Assistance
Benefits:
- Consultation & revisions included.
- Regular progress updates.
- Timely delivery always guaranteed.
- Formatting in APA, MLA, etc.
Premium Add-On: Plagiarism Report
Ensure your content’s originality with an optional plagiarism report using Turnitin or Grammarly Premium — available as an add-on upon request.
Get in Touch with Us
Have questions or need assistance? Fill out the form and our team will get back to you promptly.
Let’s Bring Your Research to Life
Get Expert Assistance for Your Thesis, Dissertation, or Academic Project
Whether you're stuck at the research proposal or need help polishing your final draft, our expert team is here to guide you every step of the way with precision, professionalism, and confidentiality.

